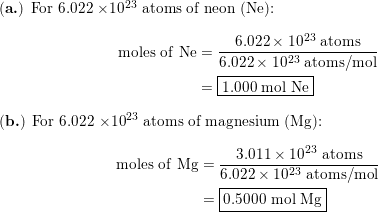Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com
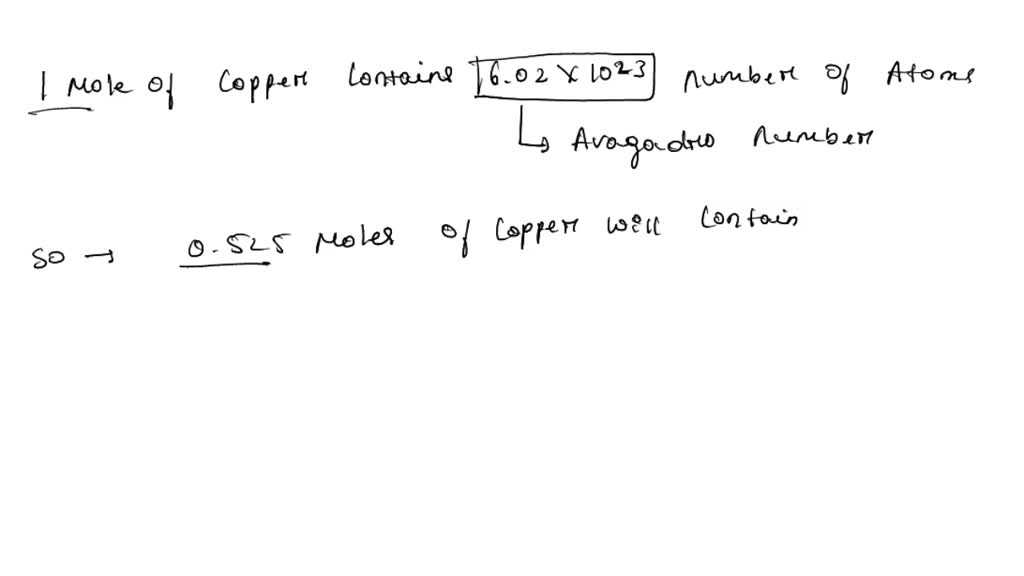
SOLVED: Knowing that Avogadro number is equal to 6.022 x 1023 atoms/mol, do the following: How many moles of iron are in 50.0 g of iron? How many moles of copper atoms
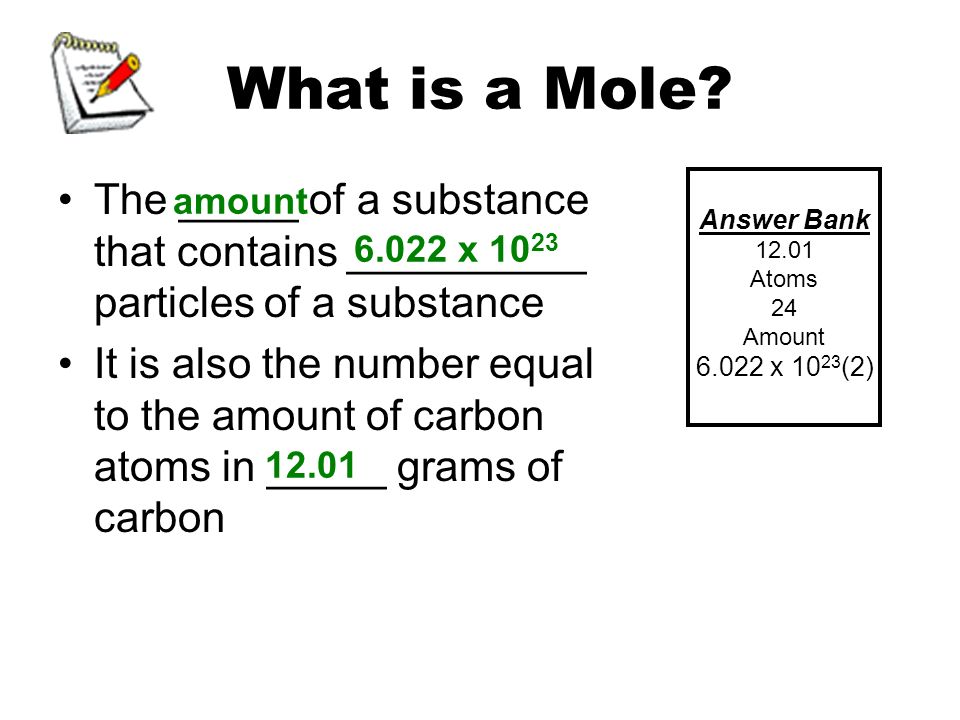
Atomic Mass and The Mole Topic: AMU's & Atomic Mass Objectives: Day 1 of 3 To learn how we define 1 amu (atomic mass unit) To learn how we derive atomic. - ppt download
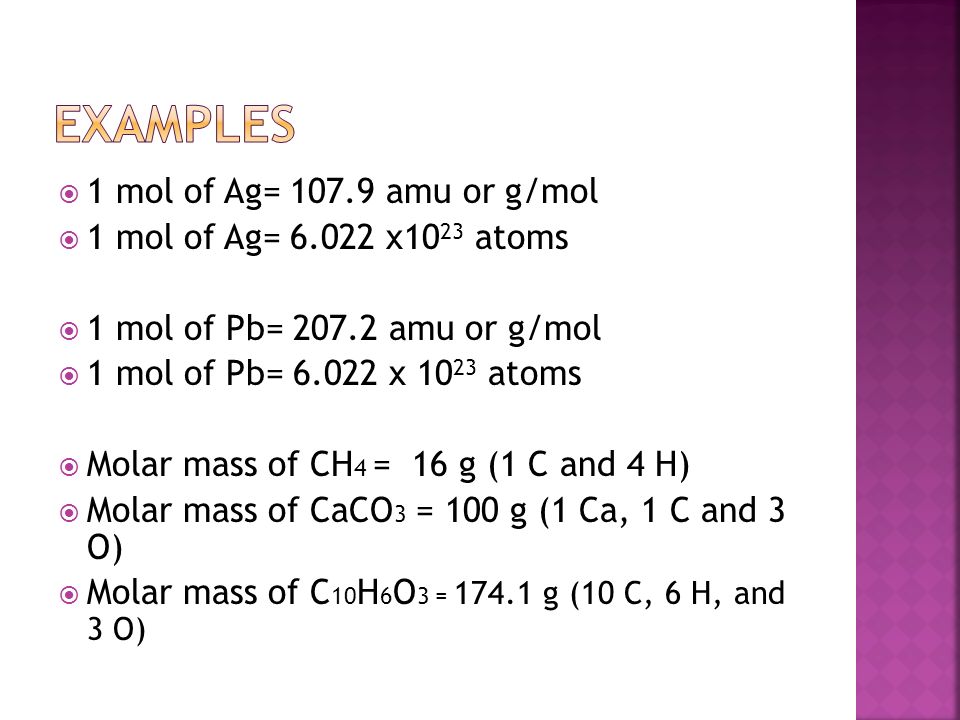
Oh My!!. Mole (mol) can be defined as the number equal to the number of carbon atoms in grams of carbon (in an chemical equation it is the coefficients. - ppt download

If Avogadro number `N_(A)` is changed from `6.022xx10^(23) mol^(-1)` to 6`.022xx10^(23) mol^(-1)`, - YouTube
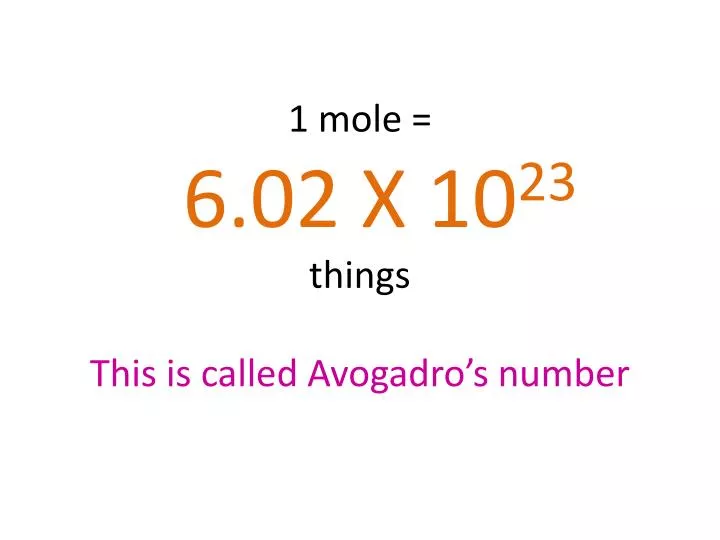

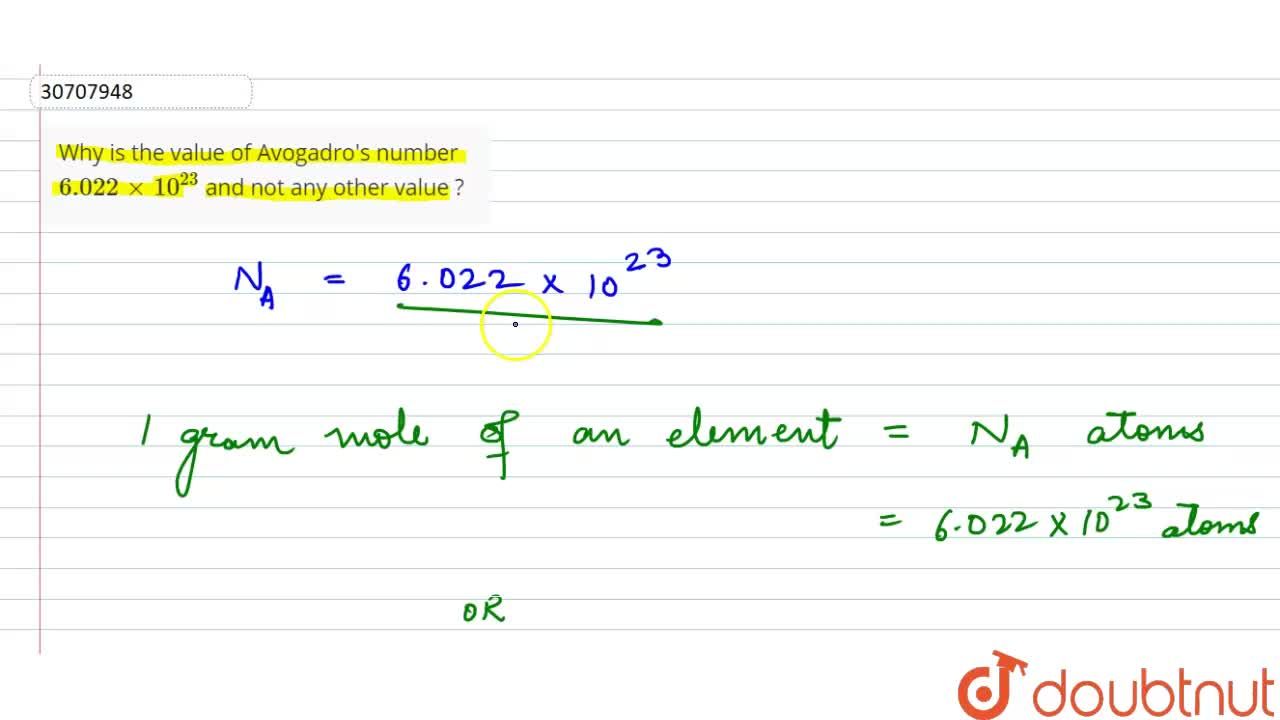
![How did Avogadro come up with the Avogadro number: [math]6.022 \times 10^{23}?[/math] - Quora How did Avogadro come up with the Avogadro number: [math]6.022 \times 10^{23}?[/math] - Quora](https://qph.cf2.quoracdn.net/main-qimg-ed17b8adf0c2f123329b850f1d223b9c.webp)