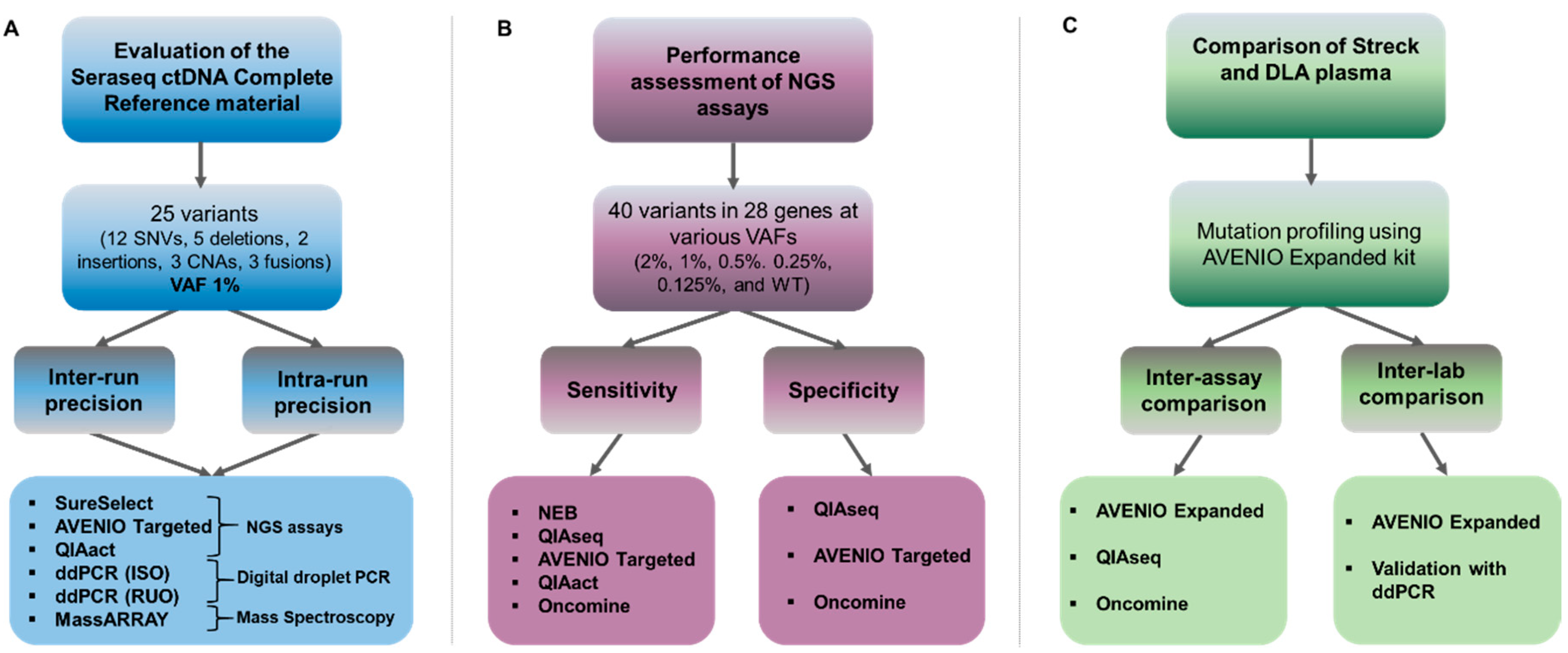
Clinical Validation of a Myeloid Next-Generation Sequencing Panel for Single-Nucleotide Variants, Insertions/Deletions, and Fusion Genes - ScienceDirect
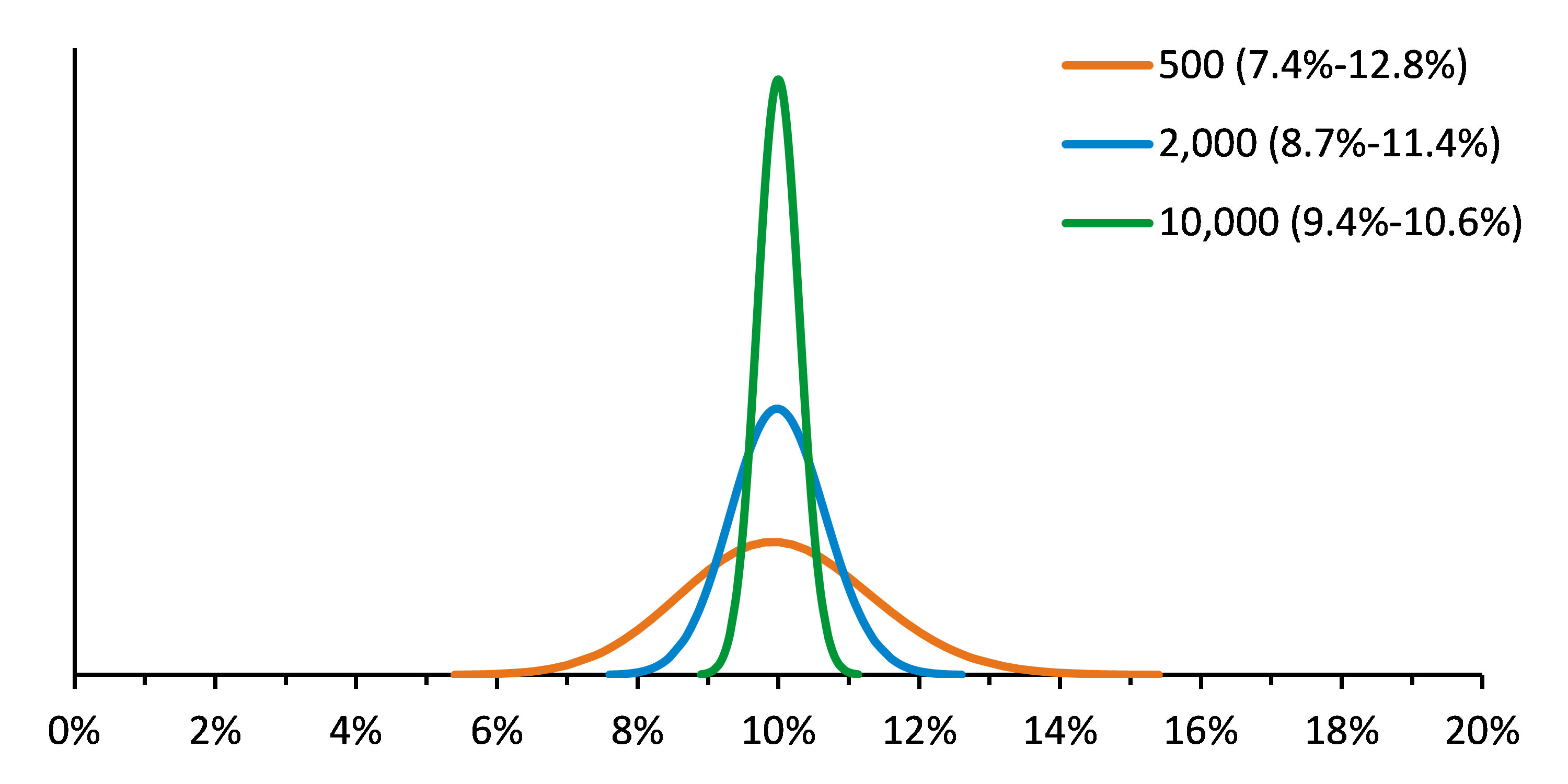
FAQ: What to do when your NGS assay fails to detect a variant contained in Seraseq Reference Materials?
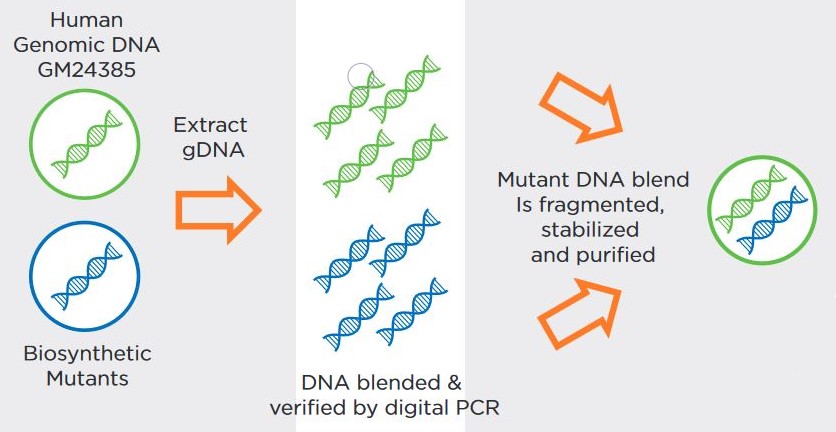
The use of Seraseq® Oncology controls & NGS validation challenges | Webinare | Technical Resources | HiSS Diagnostics